Li-ion batteries are reportedly the exception to the rule when it comes to chemical reactions while charging and discharging.
As part of the ion movement between the anode and cathode, battery experts discuss energy moving into and out of the battery.
This assertion has some validity, but if the scientists were entirely correct, the battery would never need to be replaced.
They attribute capacity fading to trapped ions, although internal corrosion and other deteriorating processes, commonly referred to as parasitic reactions on the electrolyte and electrodes, continue to play a part in all battery systems.
As a voltage-limiting device, the Li-ion charger is comparable to the lead-acid system.
With Li-ion, there is no trickle or float charge at full charge, a greater voltage per cell, and tighter voltage tolerances.
Lead acid manufacturers are less particular about the proper setup for Li-ion cells since Li-ion cannot tolerate overcharging, but lead acid allows some flexibility in terms of voltage cut off.
It is untrue that there is a “wonder charger” that uses pulses and other tricks to extend battery life and increase capacity.
Since Li-ion only takes what it can absorb, it is a “clean” system.
Cobalt-Blended Li-Ion Battery Charging
Li-ion batteries using the conventional cobalt, nickel, manganese, and aluminum cathode materials generally charge to 4.20V/cell. +/-50mV/cell is the permitted range.
High capacity Li-ion batteries may reach 4.30V/cell and higher, while certain nickel-based versions charge to 4.10V/cell.
Increasing the voltage boosts capacity, but exceeding the limit strains the battery and jeopardizes safety.
Overriding the specified voltage is prohibited by protection circuits incorporated into the battery pack.
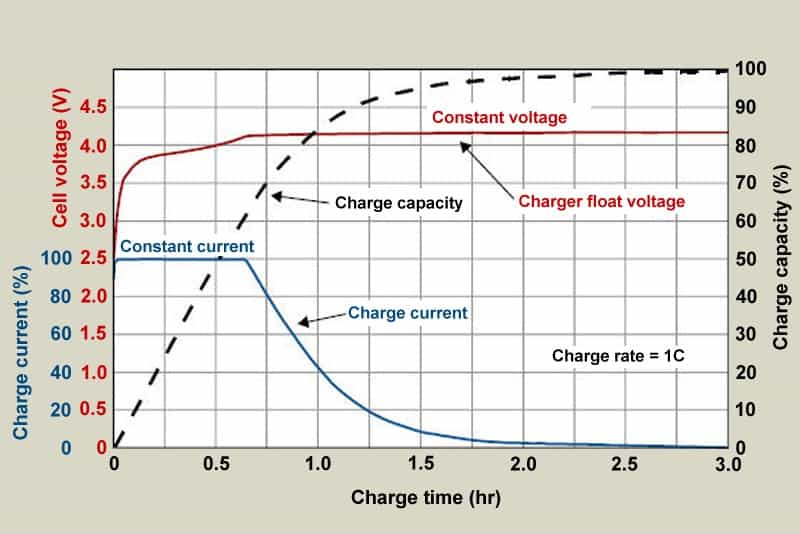
Figure 1: As lithium-ion progresses through the phases for constant current and topping charge, the voltage and current signature are shown in Figure 1.
When the current falls to between 3 and 5 percent of the Ah rating, the battery is fully charged.
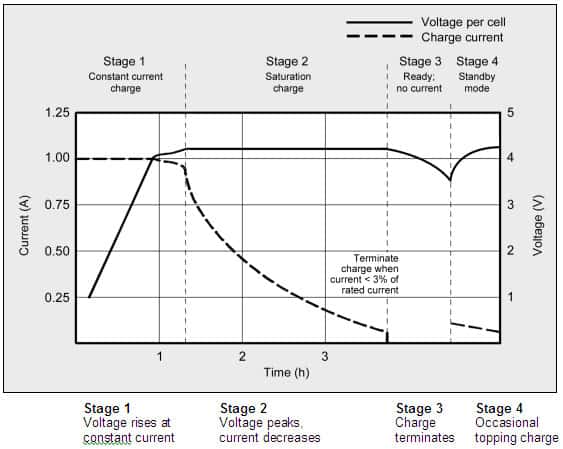
As soon as the current reaches a certain level, Li-ion has finished charging.
Some chargers apply a topping charge in place of a trickle charge when the power lowers.
An Energy Cell should be charged at a rate of between 0.5 and 1C, taking two to three hours in total.
The majority of Power Cells can handle a higher charge C-rate with minor stress, although the manufacturers of these cells advise charging at 0.8C or less to increase battery life.
The cell stays cold throughout charging, and the charge efficiency is about 99 percent.
When achieving full charge, certain Li-ion packs may suffer a temperature increase of roughly 5 °C (9 °F).
The protective circuit or increased internal resistance may be to blame for this.
If the battery or charger temperature increases by more than 10°C (18°F) while being charged at a moderate pace, stop using it.
When the battery achieves the voltage threshold and the current lowers to 3% of the rated current, the battery is fully charged.
A battery is also regarded as completely charged if the current reaches a plateau and stops declining.
This situation could be caused by elevated self-discharge.
The full-charge condition is not significantly hastened by increasing the charge current.
The saturation charge will take longer even if the battery reaches the voltage peak more quickly.
Stage 1 is shortened with a greater current, while Stage 2’s saturation takes longer.
However, a short charge at a high current will quickly fill the battery to roughly 70%.
Unlike lead acid, which must be completely charged before use, lithium does not need or prefer this.
In actuality, it is preferable to avoid a complete charge due to the battery’s stress caused by a high voltage.
Longer battery life may be achieved by lowering the voltage threshold or doing away with the saturation charge completely, but this shortens runtime.
Consumer product chargers are designed to operate at their maximum capacity and cannot be modified; a longer service life is considered less significant.
A lithium-ion battery may be charged in one hour or less using the streamlined “charge-and-run” approach, which skips Stage 2 saturation charge, using certain less expensive consumer chargers.
When the battery passes the Stage 1 voltage threshold, the word “Ready” displays.
Currently, the state-of-charge (SoC) is at 85%, which may be enough for many users.
In order to increase battery life, certain industrial chargers intentionally reduce the charge voltage threshold.
Table 2 shows the predicted capacities with and without a saturation charge when charged to various voltage thresholds.
| Charge V/cell | Capacity at cut-off voltage* | Charge time | Capacity with full saturation |
|---|---|---|---|
| 3.80 | ~40% | 120 min | ~65% |
| 3.90 | ~60% | 135 min | ~75% |
| 4.00 | ~70% | 150 min | ~80% |
| 4.10 | ~80% | 165 min | ~90% |
| 4.20 | ~85% | 180 min | 100% |
The capacity is increased by around 10% when full saturation is added at the designated voltage, although the high voltage causes additional stress.
The voltage increases fast when the battery is initially charged.
Similar to pulling a weight with a rubber band and creating a lag, this behavior.
When the battery is nearly completely charged, the capacity will soon catch up (Figure 3).
All batteries have this charging property.
The rubber-band effect will increase with a greater charge current.
The impact is amplified when a cell with high internal resistance is charged or when the temperature is low.
Similar to using a rubber band to hoist a hefty weight, the capacity lags behind the charge voltage.
It is impossible to estimate SoC by reading the voltage of a charging battery; a better indication is the open circuit voltage (OCV), which may be measured after the battery has rested for a few hours.
Li-active ion’s material and OCV are both impacted by temperature, as they are with all batteries.
By using coulomb counting, the SoC of smartphones, laptops, and other devices is calculated.
Overcharge can’t be absorbed by Li-ion.
The charge current has to stop after the battery is completely charged.
A constant trickle charge would endanger safety and plat the metallic lithium.
Keep the lithium-ion battery’s peak cut-off as brief as feasible to reduce stress.
The battery voltage starts to fall when the charge is finished.
Thus, the voltage stress is reduced.
The open circuit voltage will eventually stabilize at a value of between 3.70V and 3.90V/cell.
It should be noted that a Li-ion battery that has been completely saturated will maintain the voltage higher for a longer period of time than one that has not.
Some chargers offer a short topping charge to lithium-ion batteries when they need to be left in the charger for operational readiness in order to make up for the little self-discharge the battery and its protection circuit incur.
When the open circuit voltage reaches 4.05V/cell, the charger may start working and then stop at 4.20V/cell.
The battery voltage is often allowed to drop below 4.00V/cell on chargers designed for operational readiness or standby mode, and they recharge to just 4.05V/cell as opposed to the full 4.20V/cell.
This lessens stress caused by voltage and increases battery life.
Some portable electronics are turned ON and rest in a charging cradle.
The parasitic load, which is the current flowing through the device, might skew the charge cycle.
Because they produce mini-cycles, battery makers advise against using parasitic loads when charging.
A laptop connected to the main AC supply is one instance where this cannot always be prevented.
The gadget could charge the battery to 4.20V/cell and then discharge it.
Because the cycles take place at the high-voltage threshold, which is often occurring at a higher temperature, the battery is under a lot of stress.
When charging, a portable gadget should be unplugged.
This enables the battery to effortlessly attain the predetermined voltage threshold and current saturation point.
By lowering the battery voltage and drawing a leakage current, a parasitic load throws off the charger by preventing the current in the saturation stage from falling low enough.
Even if a battery is completely charged, the environment will still force it to continue charging, which will put it under stress.
Pricing Non-Cobalt-Blended Materials Li-Ion
Li-phosphate (LiFePO) is an outlier, having a nominal cell voltage of 3.20V and charging to 3.65V, while conventional lithium-ion has a nominal cell voltage of 3.60V.
The Li-titanate (LTO), which has a nominal cell voltage of 2.40V and charges to 2.85V, is a relatively recent technology.
These Li-ions do not contain cobalt, hence their chargers cannot be used with standard 3.60-volt Li-ion batteries.
To identify the systems and give the proper voltage charging, provisions must be provided.
A 3.60-volt lithium battery would not get enough charge in a charger designed for Li-phosphate, whereas a Li-phosphate in a standard charger would result in overcharging.
Lithium-Ion Battery Overcharging
Lithium-ion performs securely within the authorized operating voltages; but, if the battery is unintentionally charged to a greater voltage than recommended, it becomes unstable.
A Li-ion battery designed for 4.20V/cell will plate metallic lithium on the anode after a prolonged charge over 4.30V.
The cathode material deteriorates, turns into an oxidizing agent, and emits carbon dioxide (CO2).
If the charge is permitted to proceed, the cell safety current interrupt device (CID) disconnects at 1,000-1,380kPa (145-200psi) as the cell pressure increases.
Should the pressure increase, certain Li-ion batteries’ safety membranes, which break open at roughly 3,450 kPa (500 psi), may ultimately cause the cell to erupt in flames.
Elevated temperature is associated with venting using a flame.
A battery that is completely charged will vent before one that is only partly charged because it has a lower thermal runaway temperature.
Authorities will require air transportation of Li-ion at a 30% state-of-charge rather than a full charge since all lithium-based batteries are safer with a lesser charge.
Shipping Lithium-based Batteries via Air (See BU-704a)
At full charge, the barrier for lithium-cobalt is 130-150 oC (266-302 oF), for nickel-manganese-cobalt (NMC), it is 170-180 oC (338-482 oF), and for lithium-manganese, it is roughly 250 oC (482 oF).
Comparable to and more stable at higher temperatures than manganese is lithium phosphate.
Not just lithium-ion batteries may be dangerous if overcharged.
Batteries made of lead and nickel have a reputation for melting down and catching fire when handled incorrectly.
All battery systems must have properly engineered charging equipment, and temperature sensing is a trustworthy guardian.
Summary
Compared to nickel-based systems, lithium-ion batteries are easier to charge.
The charging circuit is simple; it is simpler to accommodate voltage and current limits than it is to analyze complicated voltage signatures that vary as the battery matures.
Li-ion does not need saturation as lead acid does, hence the charging process might be irregular.
This provides a significant benefit for the storage of renewable energy sources like solar panels and wind turbines, which are not always able to completely charge the battery.
The charger is made much simpler by the lack of trickle charging.
Li-ion batteries do not need an equalizing charger, like lead acid batteries do.
The majority of commercial and consumer Li-ion chargers completely charge the battery.
They don’t have changeable end-of-charge voltages, which would reduce the end charge voltage and accept a shorter runtime to extend the service life of Li-ion batteries.
Device makers worry that adding this option will make the charging more difficult.
Electric cars and satellites that forgo a complete charge in order to have a lengthy service life are exceptions.
Simple Instructions for Lithium-Based Battery Charging
- To enable the current to drop unimpeded during saturation, turn off the device or disconnect the load that is charging. The charger gets confused by a parasitic load.
- charge in a comfortable environment. Never charge in a frigid environment.
- Fully charging lithium-ion is not necessary; a partial charge is preferable.
- The battery may not be completely charged when the “ready” indication appears, and not all chargers provide a full topping charge; a fuel gauge reading of 100 percent charge can be a deception.
- If the battery becomes too heated, stop using the charger and/or the battery.
- Before storage, give an empty battery a charge (40–50% SoC is best).